【色质谱实验室:陈银娟博士 撰文】
【仪器设备:液相色谱相关仪器】
【地点:4号楼219室 色质谱实验室】
液相色谱组成——流动相储液瓶及流动相(一)
追溯液相色谱的发展历史,最早最经典的液相色谱实验当属俄国植物学家茨维特的叶绿色提取实验:将植物色素提取液从一根装有碳酸钙吸附剂的玻璃管顶端加入,然后用纯石油醚进行淋洗,随着石油醚往下流动,不同色素逐渐分开形成色带。液相色谱技术发展到今天,商业化的色谱仪已广泛用于分析混合物,但无论仪器设备如何发展,现代色谱仪的组成仍源于茨维特实验。液相色谱仪主要包括储液瓶、泵、进样器、色谱柱、检测器、数据处理系统(图1)本专题将按色谱仪的基本组成,简要介绍各部分的工作原理及注意事项。

图1. 液相色谱结构图
Figure 1. Diagram of liquid chromatography
1. 储液瓶
储液瓶用于盛放流动相(图2),规格有25 ml、500 ml、1000 ml、2000 ml甚至更大。储液瓶通常为高硼硅玻璃材质,也有特殊情况,比如离子色谱使用聚氯乙烯储液瓶以避免引入金属离子干扰。存储特殊的流动相时,储液瓶的颜色也会不同。比如水相,常用棕色瓶进行避光,以降低细菌生长速度。
开孔储液瓶瓶盖是与储液瓶配套使用的,流动相传输管穿过瓶盖上的孔插入储液瓶内。瓶盖主要包括三部分:聚丙烯外壳、硅胶防漏垫圈和四氟乙烯白色内盖,该类材质需具有良好的抗腐蚀、耐高温性能。瓶盖有单孔、双孔、三孔等规格,用于插入不同数目的管路(图2b),管路与孔之间存在一定间隙或有多余的孔用于排气,以防止瓶内负压过低,导致流动相不能顺畅输出到泵体。
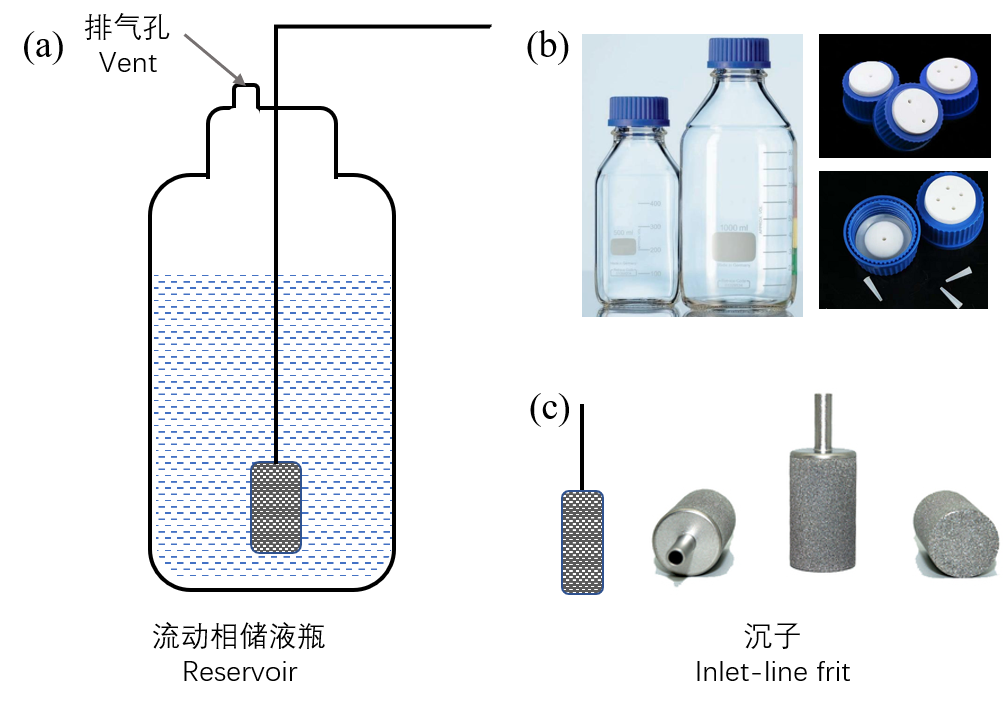
图2. 流动相储液瓶
Figure 2. Mobile-phase Reservoir
洁净度是储液瓶最基本也是最重要的标准。不干净的储液瓶会引入杂质,干扰分析结果,甚至将导致后续流动相管路堵塞,诱导泵体故障。因此,储液瓶需要定期按标准玻璃器皿清洗流程进行清洗,以确保流动相质量。主要过程如下:(1)浸酸:将储液瓶浸泡于重铬酸钾、浓硫酸、蒸馏水的清洁液中,时间不少于4小时。清洁液具有很强的氧化作用、去污能力强,能有效去除微生物和微量杂质;(2)冲洗:先用自来水充分冲洗,火吸管等冲洗10分钟,瓶内灌满、倒掉,反复20次以上;再用去离子水冲洗,不留死角,瓶内灌满、倒掉,反复10次以上,然后用超纯水进行充分漂洗和润洗,最后晾干或烘干备用。清洗液配比如下表:
表1. 不同酸度的重铬酸钾清洗液配比
Table 1. Composition of cleaning solution
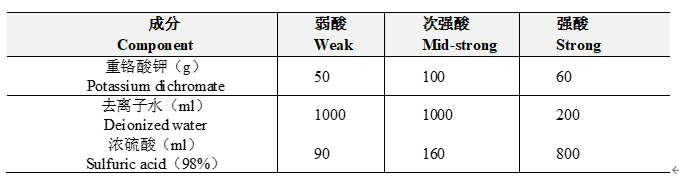
注意事项:清洗液使用耐酸抗氧化性塑料容器存储;将重铬酸钾倒入水中,搅拌溶解,然后缓慢加入浓硫酸,由于浓硫酸密度大,且遇水放出大量热,操作切勿过急,严禁将重铬酸钾溶液倒入浓硫酸中。
上述清洗方法,需进行充分的冲洗和润洗,否则可能会引入较多杂质。笔者所在的色质谱实验室,流动相更换频率较高,在储液瓶中盛满有机相和超纯水的混合溶液(比如10%-20%有机相),超声半小时再进行超纯水清洗,可达到简单清洗的目的。储液瓶除定期清洗外,平时操作应养成良好习惯,比如:勤换水相;更换流动相,先润洗再更新,禁止在剩余流动相中直接添加新的流动相;瓶盖上多余不用的孔,可用堵头堵紧或用铝箔封住储液瓶口,防止灰尘落入。但是原则上不建议使用封口膜封住储液瓶口,因为流动相可能会溶出封口膜中的聚合物成分产生杂质。
2. 过滤沉子
过滤沉子位于流动相管路末端(图2a),在重力作用下使流动相管路没入储液瓶底部。沉子是表面具有1 μm、2 μm、5 μm、10 μm、20 μm等各种孔径规格的吸滤头,常用规格为2 μm和10 μm,能有效防止已经进入储液瓶内的灰尘进入后续液相系统。储液瓶一般高于泵体50 cm以上,基于虹吸作用,流动相能快速经过沉子。市场上基本的沉子有不锈钢、陶瓷、Peek等其他惰性材质(图2c)。
过滤沉子也需定期进行超声清洗。用于反相色谱的沉子,先用超纯水加热超声15分钟以除去沉子上的盐分,再用HPLC级的异丙醇超声15分钟除去有机物,最后用HPLC级甲醇超声15分钟除去难溶物。用于正相色谱的沉子,超声使用的溶剂依次为乙醇-超纯水-异丙醇-乙醇,超声时间为15分钟左右。
3. 流动相
流动相是影响色谱分离的关键因素之一,高纯度、无微粒的流动相是液相色谱分析最重要的标准之一。满足这一标准,最简单的方式是尽可能使用高质量的溶剂,高效液相色谱纯(HPLC)级别是液相色谱的基本要求,对于液质联用仪器,由于质谱具有很高的灵敏度,要求使用质谱纯(LC-MS)级别的溶剂。
3.1配制 液相流动相可分有机相和水相,为保证流动相配比的准确性和质量,配制流动相时需注意以下事项:
流动相配置过程中使用的量筒等玻璃器皿需预先清洗干净,有机相和水相使用的量筒最好分开使用;
尽可能使用HPLC或LC-MS纯度试剂,如加入甲酸、乙酸或氨水类添加剂,也尽量使用高纯试剂,盖上盖子摇匀;
含缓冲盐的流动相,需准确称量缓冲盐,并置于容量瓶中溶解;
对pH有要求的流动相,加入酸、碱进行pH值调节,并测量pH值;
流动相需经过滤和脱气处理;
配制好的流动相,需注明流动相成分及配制日期。
3.2过滤 进口商业化的HPLC试剂在分瓶之前已经过滤膜过滤(一般为0.22 μm),Milli-Q 超纯水在纯化过程的最后一步,也会经过0.22μm滤膜。此类HPLC纯度的溶剂可以直接使用,无需再进行过滤。如果在上述流动相中添加其他非HPLC级别的试剂或者缓冲盐,使用前强烈建议过0.22 μm滤膜。
流动相过滤装置见图3,主要包括:滤杯,滤膜,砂芯,接收瓶,真空泵。流动相盛放于滤杯中,在真空用下,经滤膜和砂芯过滤,最后被接收瓶接收。过滤装置使用的玻璃器皿或连接管要求为惰性材质,避免引入更多杂质。滤膜常用孔径为0.22 μm和0.45 μm,根据流动相性质差异,滤膜又可分水相滤膜和有机相滤膜,以确保过滤过程中,滤膜成分不被流动相溶解带入更多杂质。
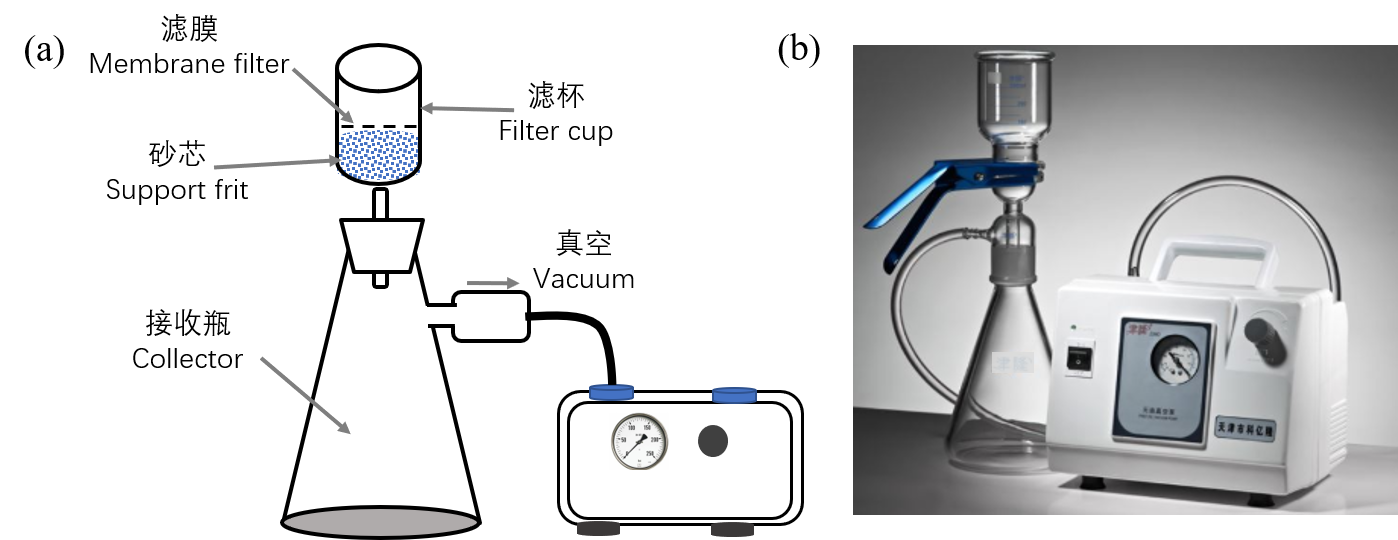
图3 流动相过滤装置图
Figure 3. Schematic of mobile phase filtration.
3.3脱气 商业化的液相色谱仪具有在线脱气功能,保证流动相进入泵前除去气泡。流动相中若含有气泡,会导致泵压增加,流动相传输不畅;对于检测器而言,气泡的存在会产生假的色谱信号,导致基线不稳;对于电化学检测器,气泡中氧气的存在,甚至导致化合物氧化、检测结果不准。因此流动相需要进行脱气处理。氦气喷雾脱气、真空脱气和在线脱气是常见的脱气方式。
3.3.1氦气脱气 氦气在流动相中溶解度小,可有效脱去流动相中的气泡。具体做法为:在流动相储液瓶中,插入一根氦气管路,没入流动相的一端连接过滤沉子,另一端连接低压力的氦气流(5 psi左右),由于氦气难溶,在其逸出液面过程中将带走流动相中溶解的空气。需要提醒的是,不同体系,使用氦气脱气也稍有不同。反相色谱,使用该方法脱气数分钟可达到目标;而正相色谱,由于流动相极易挥发,需缩短脱气时间或通过其他方式脱气。常规的液相分析仪器,使用氦气脱气较少,在制备液相色谱中,由于管路粗,流动相流速大,流动相中易含有空气,氦气脱气较为普遍。色质谱实验室正反相制备色谱仪,均采用氦气补入进行脱气。
3.3.2真空脱气 流动相置于真空环境10-15分钟,可有效脱去流动相中60-70%的溶解气体。在图3中,流动相过滤结束,接收瓶用惰性橡皮塞封口,真空泵继续工作数分钟,即可实现流动相脱气。实验室中将整个流动相储液瓶放在超声波清洗仪中进行超声,也可脱气。
3.3.3在线脱气 在线脱气是最为普遍的一种脱气方式,商业化的液相设备都装有在线脱气机,位于高压混合器或者比例阀之前(图4a)。在线脱气机的工作原理如图4b所示:流动相经过聚合物管路,该管路能选择性让气泡通过,而流动相不通过,整个管路置于真空腔体中,由于压力变化,溶解在流动相中的气泡将被拉出聚合物管道。与氦气脱气相比,在线脱气效率较低,但成本较低。

图4在线脱气机原理图
Figure 4. Schematic of on-line degassing
本篇主要介绍液相色谱仪流动相储液瓶、过滤沉子的作用及清洗,重点介绍了流动相配制过程中的注意事项以及流动相过滤、脱气的方法和原理。看似简单的流动相,其实也有大文章,它的质量好坏,直接影响后续分析结果。后续笔者将陆续更新色谱泵、色谱柱、检测器等相关原理和组成的介绍,敬请期待。
Components of Liquid Chromatography - Reservoir and Mobile Phase (I)
To trace the development history of liquid chromatography, the earliest and most classic liquid chromatography experiment is the chlorophyll extraction experiment by the Russian botanist Tswett: add the plant pigment extract from the top of a glass tube containing a calcium carbonate adsorbent, and then rinse with pure petroleum ether. As the petroleum ether flows down, different pigments can gradually be separated, forming a color band. Presently, with the technology development, commercial chromatographs have been widely used to analyze mixtures, but no matter how the instrument is developed, the composition of modern chromatograph still originates from Tswett’s experiment. The liquid chromatograph mainly includes reservoir, pump, sampler, column, detector and data processing system (Figure 1). This topic will briefly introduce the working principle and precautions of all these parts.
1 Reservoir
Reservoir is used to contain the mobile phase, and the specifications includes 25 ml, 500 ml, 1000 ml, 2000 ml and even larger (Figure 2). Most reservoirs are made of glass except some special cases. For example, polyvinyl chloride reservoir is used for ion chromatography to avoid the introduction of metal ions. The reservoir color may vary sometimes. For the water phase, brown bottles are often used to protect from light, reducing the growth rate of bacteria. Open-hole caps are coupled with reservoir. The mobile phase transmission tube is inserted into reservoir through the hole. The cap consists of three parts: polypropylene shell, a silicone leak-proof gasket, and tetrafluoroethylene inner cap. The material should have good resistance properties of corrosion and high temperature. Single hole, double holes, three holes and other specifications can be used for different tubes. Gap between the tube and hole or other free holes are for exhausting.
Cleanliness is the most basic and important criterion for reservoir. The dirty reservoir will introduce impurities, interfere with the analysis results, and even cause the subsequent tubes blocked, or induce pump malfunction. Therefore, it is wise to wash reservoir regularly according to standard glassware cleaning procedures to ensure the quality of the mobile phase. The main process is as follows: (1) Acid immersion: immerse the reservoir with cleaning solution of potassium dichromate, concentrated sulfuric acid, and deionized water for over four hours. The cleaning solution has strong oxidation effect and decontamination ability, which can effectively remove microorganisms and trace impurities; (2) Rinse: Rinse thoroughly with tap water first for 10 minutes, fill and pour the bottle more than 20 times; rinse with deionized water thoroughly. Fill and pour the bottle, repeat it more than 10 times, then rinse thoroughly with ultrapure water, and finally dry the reservoir. The cleaning solution can be prepared according to Table 1.
The above cleaning procedure need thorough rinses, or more impurities may be introduced. In our chromatography and mass spectroscopy lab, the mobile phase is frequently replaced. Simple reservoir cleaning is achieved by ultrasound washing for half an hour. The reservoir is filled with a mixed solution of organic phase and ultrapure water. Besides the washing, good habits should be developed during normal operations, such as frequent water phase changes; the mobile phase needs to be rinsed and renewed. It is forbidden to add the new to the remaining. Unused holes can be closed with plugs or sealed with aluminum foil to prevent dust. It is not safe to use film products, because the mobile phase may dissolve the polymer components in film and produce impurities.
2. Inlet-line frit
The inlet-line frit is at the end of the mobile-phase tube (Figure 2c), and the tube is submerged into the bottom of reservoir by gravity. The frit is a suction filter with various pore sizes of 1 μm, 2 μm, 5 μm, 10 μm, and 20 μm on the surface. And frits of 2 μm and 10 μm are the commonly used. The prime function of frit is to prevent the dust from entering the subsequent LC parts. The reservoir locates 50 cm higher than pump, so the mobile phase can quickly pass through the frit based on the siphon effect. The available frits are made of stainless steel, ceramic, peek and other inert materials.
Regular ultrasonic cleaning is required for frit. Frit used for reversed-phase chromatography should be firstly sonicated with ultrapure water for 15 minutes to remove the salt, and then sonicated with HPLC-grade isopropanol for 15 minutes to remove the organic. Finally, it should be sonicated with HPLC-grade methanol for 15 minutes to remove insoluble compounds. For frit used in normal phase chromatography, the solvent used for ultrasound is ethanol-ultrapure water-isopropanol-ethanol.
3. Mobile Phase
Mobile phase is one of the key factors affecting chromatographic separation. High-purity, particulate-free mobile phase is one of the most important standards. The simplest way to meet this standard is to use high-quality solvents as much as possible. High-performance liquid chromatography (HPLC) grade solvent is the basic requirement of liquid chromatography. For LC/MS instruments, LC-MS grade solvent is required due to the high sensitivity of mass spectrometry.
3.1 Preparation Mobile phase can be divided into organic phase and aqueous phase. To ensure the accuracy and high quality of the mobile phase, the following precautions must be taken when preparing the mobile phase:
1) Glassware should be cleaned in advance. The graduated cylinders used for the organic phase and the aqueous phase should be used separately.
2) Use HPLC or LC-MS purity reagents as much as possible. High-purity additives of formic acid, acetic acid, etc., also required;
3) Buffer salt should be accurately weighed and dissolved in a volumetric flask;
4) Adding acid and base to adjust the pH value, and measure the pH value;
5) The mobile phase also needs to be filtered and degassed;
6) The mobile phase composition and the preparation date must be indicated.
3.2 Filtration Imported commercial HPLC reagents are filtered through a membrane filter (usually 0.22 μm) before separation. Milli-Q ultrapure water is also filtered through a 0.22 μm filter in the final step of purification. These HPLC-purity solvents can be used directly without further filtration. If other non-HPLC grade reagents or buffer salts are added to the above mobile phase, filtration with 0.22 μm filter is strongly recommended before using.
The mobile phase filtration device is shown in Figure 3. It consists with filter cup, membrane filter, support frit, collector, vacuum pump. The mobile phase is contained in a filter cup, and is filtered through membrane filter and support frit under vacuum, and finally collected by collector. Inert materials are required for the glassware or tubes to avoid introducing more impurities. The common pore sizes of membrane filter are 0.22 μm and 0.45 μm. According to the property differences of mobile phases, water-phase filters and organic-phase filters should be selected.
3.3 Degassing Commercial liquid chromatography has an on-line degassing function to ensure that the mobile phase removes air bubbles before entering the pump. If air bubbles exist, the pump pressure will increase and the mobile phase will not be transmitted smoothly. For the detector, air bubbles usually cause spurious peaks. For electrochemical detectors, the presence of oxygen even causes compound oxidation. Therefore, the mobile phase needs to be degassed. Helium spray degassing, vacuum degassing, and online degassing are common degassing methods.
3.3.1 Helium sparging Helium has a low solubility in the mobile phase and can effectively remove bubbles. The specific process is: insert a helium gas tube into reservoir with one end connected to frit, and the other end connected to a low-pressure helium gas stream (about 5 psi). It should be reminded that helium degassing is slightly different for different type systems. For reversed-phase chromatography, this method can be used for several minutes to degas; however, for normal-phase chromatography, the degassing time needs to be shortened or by other means as the mobile phase is extremely volatile. Compared with LC or LC-MS for analysis, helium sparging is more common in preparative liquid chromatography due to the thick tube and large mobile phase flow rates. In our chromatography and mass spectrometry laboratory, helium degassing is applied in two preparative chromatography instruments.
3.3.2 Vacuum degassing The mobile phase is in vacuum for 10-15 minutes, 60-70% of the dissolved gas can be effectively removed. In Figure 3, after filtration, sealed the collector with an inert rubber stopper, degassing is carried out via the vacuum pump working for several minutes. Sonication also enable degassing.
3.3.3 On-line degassing On-line degassing is the most common type of degassing. Commercial liquid chromatography is equipped with an on-line degasser, located prior the high-pressure mixer or proportional valve (Fig.4a). The principle is shown in Figure 4b. The mobile phase passes through the polymer tubing, which allow air bubbles to pass except the solvent. The tubing is placed in a vacuum chamber. Due to the pressure change, bubbles dissolved in the mobile phase will be pulled out. Compared with helium sparging, on-line degassing is less efficient, but the lower cost makes it the preferred degassing method.
Reservoir and frit are briefly introduced herein, including its functions and cleaning. More words are relative with mobile phase preparation, filtration and degassing. Please keep in mind: a great deal of knowledge is concealed in the common utilization of simple mobile phase. The author will continue to update the introductions to chromatographic pump, chromatographic column, detector and other related topics in the coming future.
参考资料/ References
Lloyd R.Snyder, Joseph J. Kirkland, John W.Dolan. Introduction to Modern Liquid Chromatography (Third Edition). A John Wiley&Sons, Inc., Publication.